![]()
Basic Research
What are Magnetotactic Bacteria?
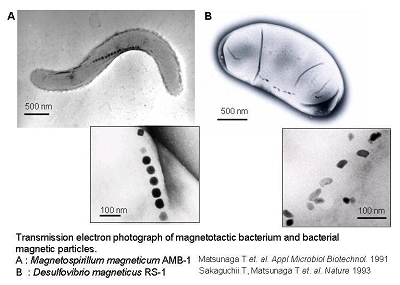
Magnetotactic bacteria are known to synthesize fine crystalline particles of magnetite (Fe3O4) known as bacterial magnetic particles (BacMPs). These naturally synthesized BacMPs are enveloped by a thin lipid bilayer and are highly uniform in shape and size ranging from 50 to 100 nm in diameter. The alignment of BacMPs in chains confers a magnetic dipole and magnetotaxis to the cells allowing bacterial orientation along geomagnetic field lines. In this laboratory, the successful isolation, cultivation and characterization of a facultative aerobic magnetic bacterium, Magnetospirillum magneticum AMB-1, has enabled us to conduct genetic manipulations to elucidate the mechanism of BacMP formation as well as to study the useful applications of BacMPs to the biotechnology field. Recently, we have also succeeded in the isolation and characterization of a sulfate-reducing magnetic bacterium, Desulfovibrio magneticus RS-1, which grows and synthesizes BacMPs only under strict anaerobic conditions. The following introduction summarizes our recent genetic and protein analyses related to the biomineralization of magnetite and the potential applications of BacMPs.
Properties of Bacterial Magnetic Particles
Transmission electron microscopy (TEM) imaging shows BacMPs are encapsulated by a biolipid membrane. This unique characteristic of BacMPs imparts superior dispersion properties in aqueous solutions in comparison with commercially available artificial magnetic particles. Using this to our advantage, sensitive and high throughput techniques for DNA detection, immunoassay and various types of enzymatic assays have been developed by using BacMPs.
Analysis of BacMP Formation Mechanism
Isolation and characterization of genes that mediate magnetite formation in bacteria are prerequisite for determining the mechanisms of magnetic particle biosynthesis. With the isolation and analysis of several proteins located on or in the BacMP biolipid membrane found in M. magneticum AMB-1, we were able to postulate the steps of BacMP synthesis. The molecular mechanism of BacMP synthesis is a multiple process, which includes vesicle formation, iron transport, and magnetite crystallization. The first event of BacMP synthesis is the formation of vesicles where the invagination of the cytoplasmic membrane is primed by a BacMP membrane-specific GTPase (Mms16) to form the intracellular vesicle. MpsA, a homolog of an acetyltransferase containing a CoA-binding motif, is also considered to be involved in this process. Once the vesicles are formed, iron is then transported into the vesicle, and further oxidized to produce magnetite. The magA gene, isolated through transposon mutagenesis which encodes for an integral membrane protein, plays an important role in iron transport into the vesicles. Finally, the magnetites are crystallized in the vesicles to complete the process of BacMP synthesis. Here, an acidic iron-binding protein (Mms6) which is tightly bound to the BacMP crystal is considered to be directly involved in the magnetite crystal formation.
With the recent completion of the whole genome sequencing of M. magneticum AMB-1, we have established a database which now allows us to conduct transcriptome and proteome analyses in order to further investigate and to understand the detailed mechanism of this highly controlled biomineralization process.
Application

Preparation of Genetically Engineered BacMPs and Its Applications
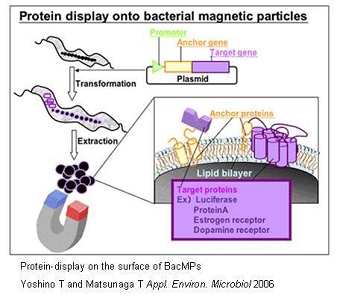
Several BacMP membrane proteins, which exist within the biolipid membrane of BacMPs can be utilized as anchor molecules, allowing a variety of functional proteins to be displayed on BacMP surface. This can be achieved by the fusion of the gene of the protein to be displayed to an anchor protein gene using genetic engineering techniques. One example would be the fusion of the firefly luciferase luc gene with the magA gene which encodes for a BacMP membrane protein. The constructed magA-luc fusion gene was then cloned into a broad host plasmid, and introduced into M. magneticum AMB-1. Extracted BacMPs from the recombinant AMB-1 cells showed luciferase activity indicating that the MagA protein acts as an anchor molecule for the site-specific introduction of functional foreign proteins. Similarly, protein A was introduced on to BacMP membrane. By applying antibody bound protein A-BacMP complexes to a chemiluminescence enzyme immunoassay, a rapid and highly sensitive diagnostic method for detecting insulin, HbA and prostate specific antigen was developed. We then employed the use of a new membrane anchor protein, Mms16, in the assembly of seven transmembrane domain proteins known as G protein-coupled receptors (GPCRs), on to BacMP membrane. The large transmembrane proteins retained their native forms as well as bioactivity, providing us with a new approach to study membrane proteins which are usually difficult to assay.
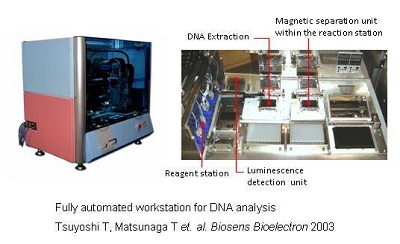
Automated Bioassay System using BacMPs
The use of BacMPs as an antibody carrier enables the separation of bound antigens from free antigens by simply applying a magnetic field. Using this special characteristic, a sandwich chemiluminescence enzyme immunoassay using antibody-conjugated to BacMPs permits the development of highly sensitive assays for insulin, HbA1c, glycated albumin and IgG. In addition, the development of a competitive chemiluminescence enzyme immunoassay allows us to detect small molecules, such as environmental pollutants, hormone and toxic detergents.